O H Bond Energy

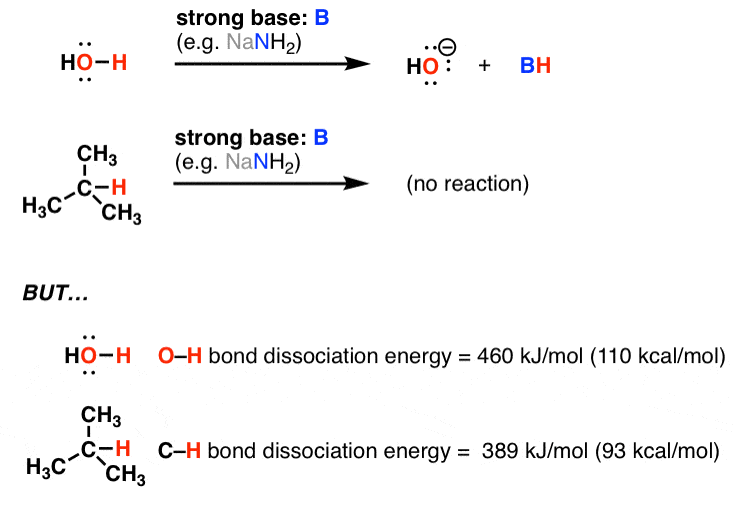
Even in modern times between 1990 and 2004 the o h bond of phenol has been reported to be anywhere from 85 8 to 91 0 kcal mol.
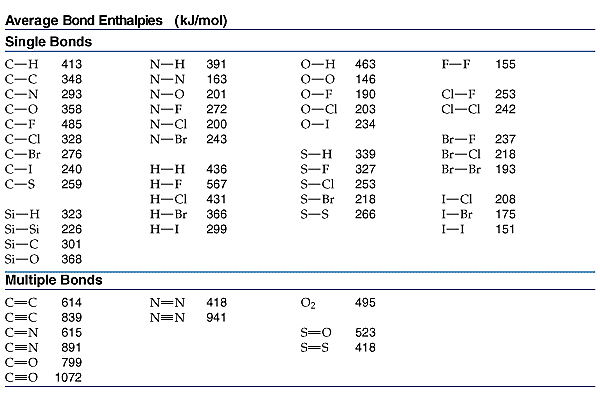
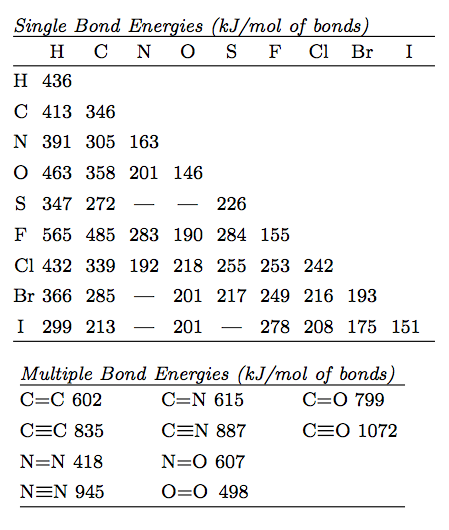
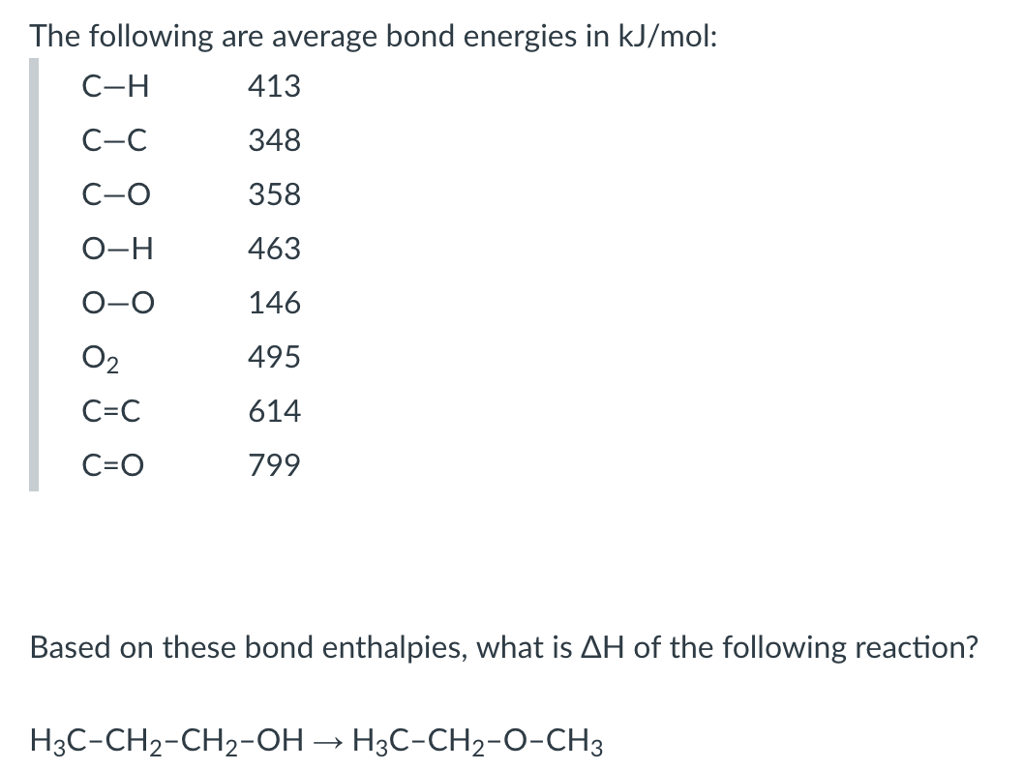
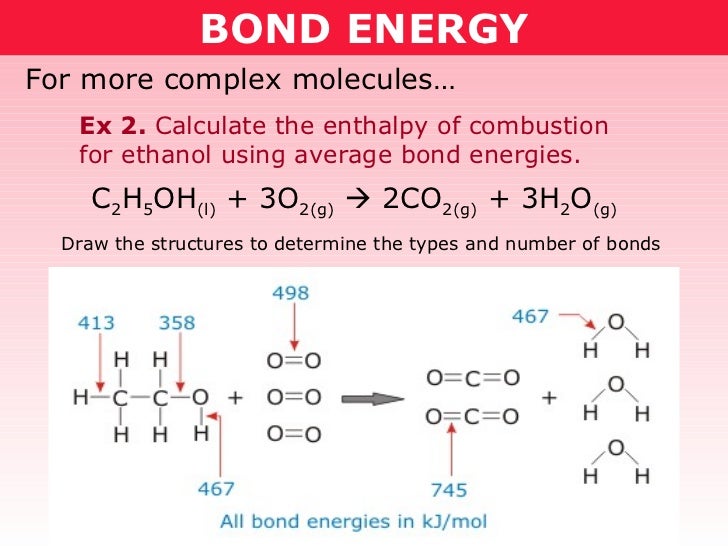
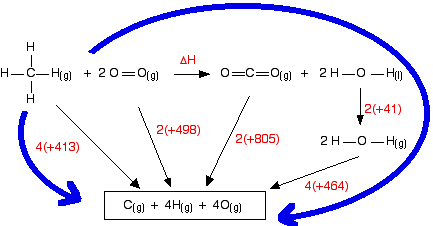
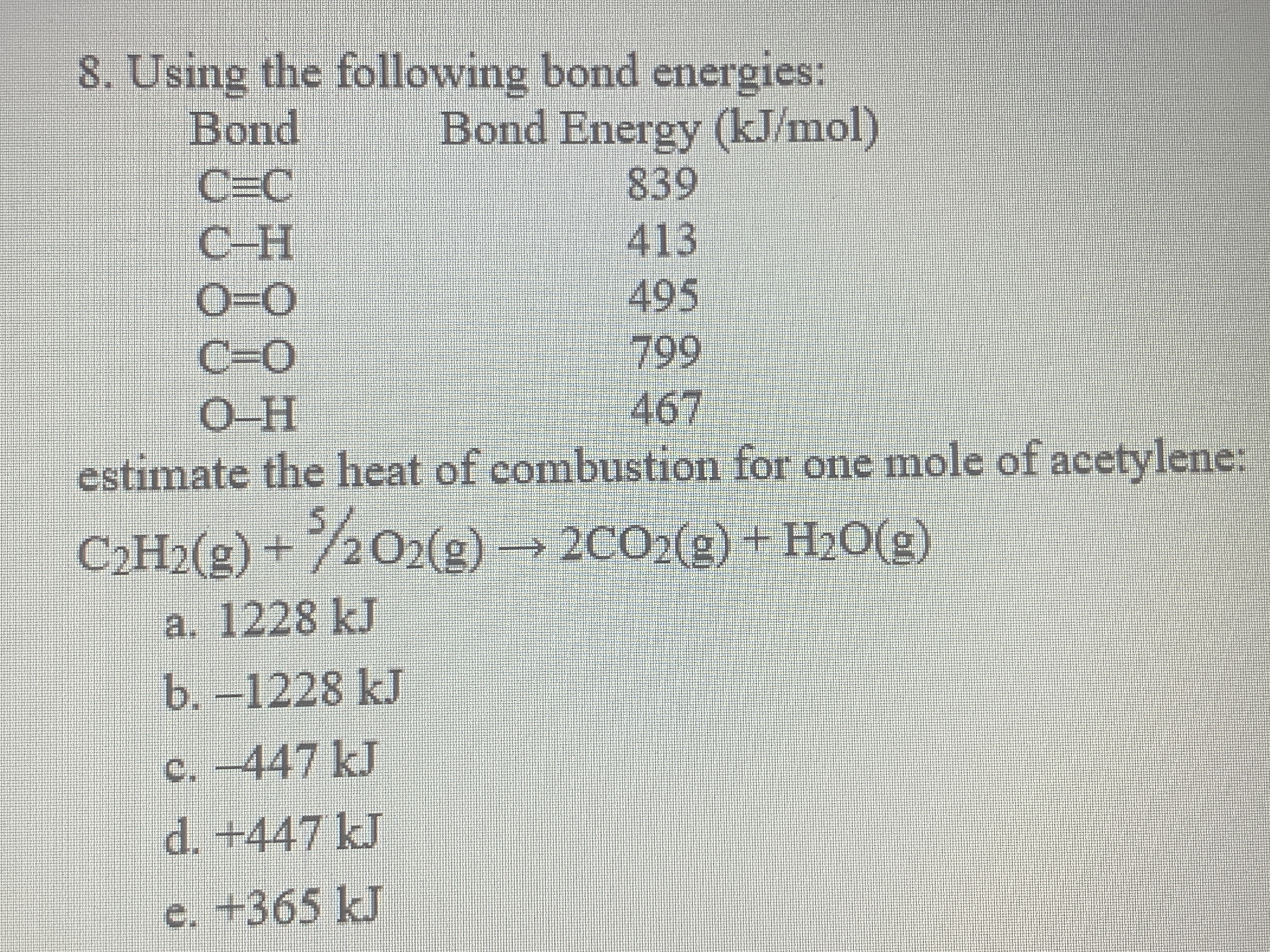
O h bond energy. 2 h h bonds are broken. The average bond energies in table t3 are the averages of bond dissociation energies. Referenced from chemistry by zumdahl 5th ed table 8 4 on page 373chemistry by zumdahl 5th ed table 8 4 on page 373. These energy values 493 and 424 kj mol required to break successive o h bonds in the water molecule are called bond dissociation energies and they are different from the bond energy.
Darwent national standard. Dh 298 h h 104 1539 1 kcal mol. Cottrell the strengths of chemical bonds 2nd ed butterworths london 1958. For example the average bond energy of o h in h 2 o is 464 kj mol.
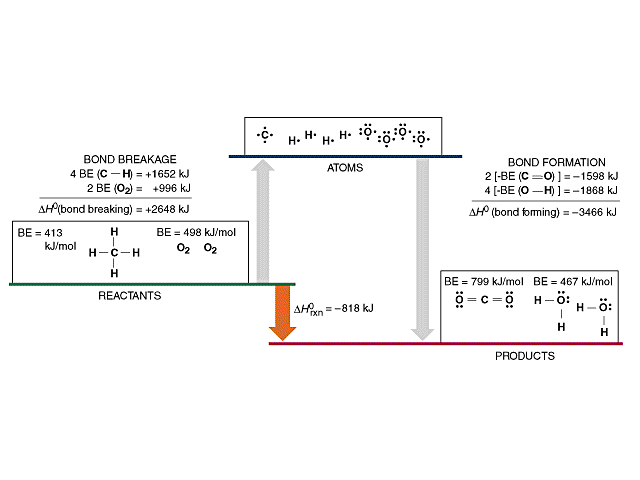
On the other hand the bond dissociation energy of h 2 at 298 k has been measured to high precision and accuracy. We have to figure out which bonds are broken and which bonds are formed. This is due to the fact that the h oh bond requires 498 7 kj mol to dissociate while the o h bond needs 428 kj mol. The strength of the relevant bonds in bond making and bond breaking processes can directly affect the overall efficiency of the process.
Bond energy kj mol h h. Many of the bond energies listed here were taken from the following sources. 1 o o bond is broken. R t sanderson polar covalence 1983 r t sanderson chemical bonds and bond energy 1976 chemical bonds and bond energy 1976.
Copper oxygen sites are known to catalyze reactions with some of the most recalcitrant c h bonds found in nature as quantified by bond dissociation free energy bdfe yet only a handful of copper bound o h bond strengths have been defined. Now we can substitute the values given into the equation. 2 o h bonds are formed per water molecule and there are 2 water molecules formed therefore 4 o h bonds are formed. The bond energy is the average of the bond dissociation energies in a molecule.