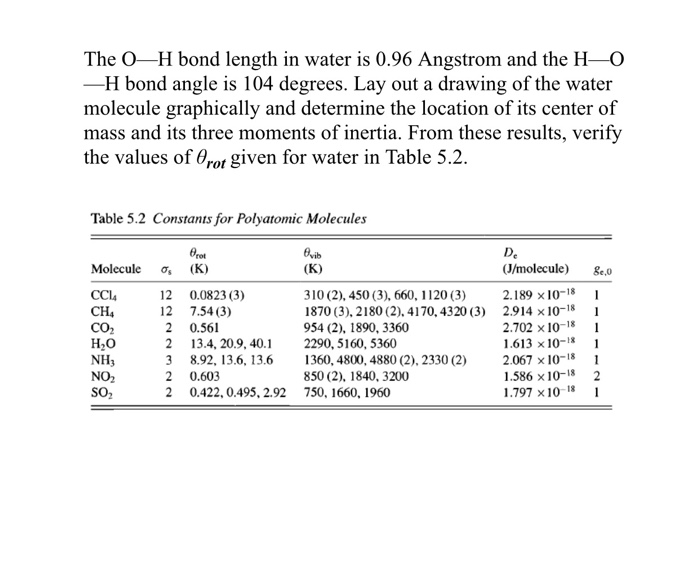
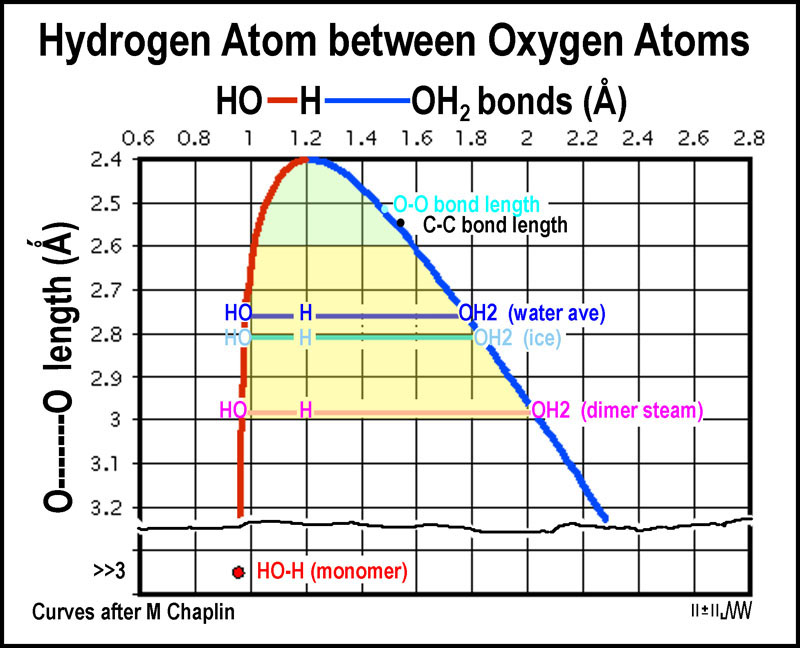
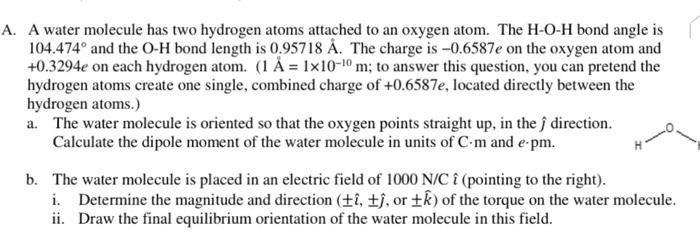
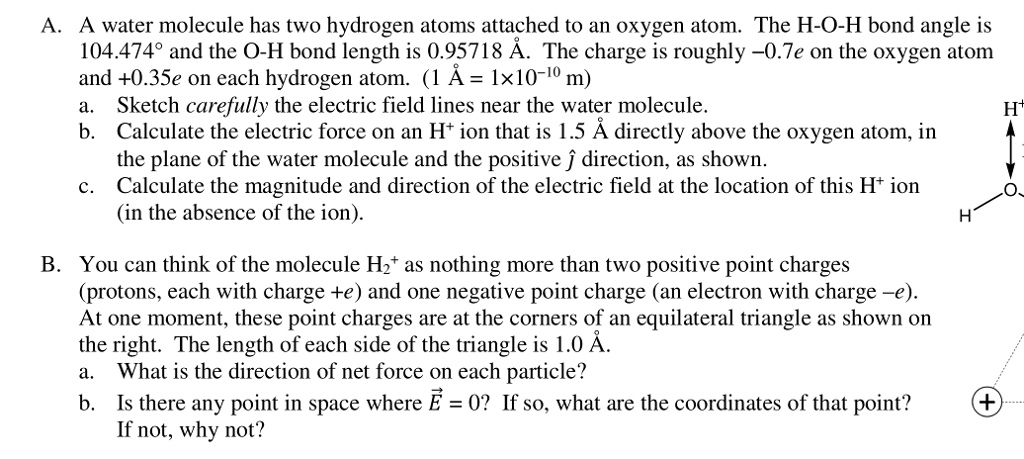
O H Bond Length In Water

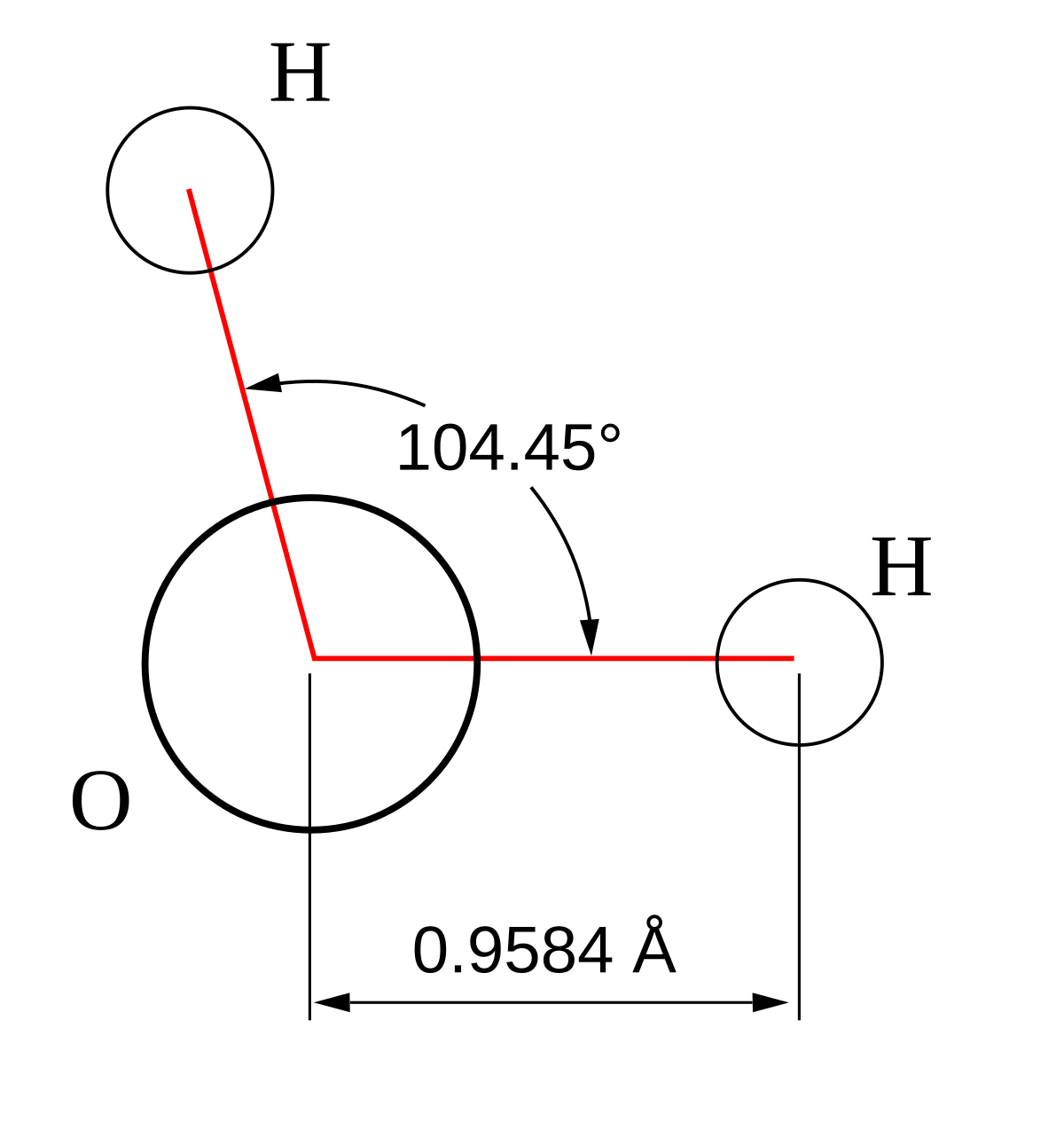
The h o bond length is about 0 96 ångström å.
O h bond length in water. For example dissociation of ho h bond of a water molecule h 2 o requires 118 8 kcal mol 497 1 kj mol. The o hbond lengths in the water molecule h2o are 0 96 å and the h o hangle is 104 5. The dipolemoment of the water molecule is 1 85 d. Its value is the oh bond length.
The dipolemoment of the water molecule is 1 85 d. The dissociation of the remaining hydroxyl radical requires 101 8 kcal mol 425 9 kj mol. The dipole moment of the water molecule is 1 85 d. The dipole moment is 1 8546 0 0006 debye.
Because an oxygen atom has a greater electronegativity than a hydrogen atom the o h bonds in the water molecule are polar with the oxygen bearing a partial negative charge δ and. The bond energy of the covalent o h bonds in water is said to be 110 3 kcal mol 461 5 kj mol the average of these values. Calculate the magnitude of the bond dipole of the o hbonds. In the gas phase.
Q 4 80 d is the charge in d units debyes not amperes. Intramolecular o h bond length and quadrupole coupling constants of water in polyelectrolyte solutions. D 0 96 å is the distance from the nucleus of the h atom to the nucleus of the o atom. Vibrational normal modes are located at 3756 cm 1 symmetricstretch 3657 cm 1 antisymmetric stretch and 1595 cm 1 bend.
The o h distance bond length is 95 7 picometres 9 57 10 11 metres or 3 77 10 9 inches. The o h bond lengths in the water molecule h20 are 0 96 a and the h o h angle is 104 5 o. A nuclear magnetic relaxation study j. Note you will need to use vector addition to do this.